Being a biologist can be rough sometimes. We’re trying to understand and work with a system that’s been changing and self-modifying itself for billions of years. Unifying principles are hard when you’re talking about living things.
But one of my touchstones to understanding life is that “Nothing in Biology Makes Sense except in the Light of Evolution”1. To comprehend the purpose of anything biological, you need to understand what pressures guided its evolution. Over the last few billion years this process has shaped every living thing - and the planet Earth.
A key insight is that organisms are not passive participants in evolution. There are a plethora of adaptations and traits that actively guide evolution, controlling how adaptive diversity is generated, spread and selected upon. Some simple examples are mutation rate, genome size and sexual reproduction. But if we dive deeper, we see that the fundamental natures of deep biological concepts - like gene regulation and genetic architecture - are optimized for evolvability.
These are meta-adaptions, traits that change how further traits are acquired, and understanding them helps us understand why life functions the way it does. If we can figure out the purpose behind evolvability adaptations, we can start mindfully engineering them to not just control an organism, but control how it evolves. This kind of knowledge will be vital as bioengineering moves towards whole-organism design.
Furthermore, being able to control evolvability unlocks new tools. Supercharging evolution would let us quickly adapt life to an incredible array of conditions, like the insides of bioreactors, polluted mines and even other planets in our solar system2.
Just as important would be ‘freezing’ a designed species to prevent evolution. We don’t want our bioproduction or carbon sequestration strains developing beyond what we’ve built them to do3. If we can one day grow a house, we don’t want that house evolving away from its blueprint - because it almost certainly won’t evolve to become a better house4.
If we as a species want to get good at engineering the self-replicating nanotechnology of life, we need to master the force that shapes its development - evolution.
Evolvability is Diversity Acquisition
Evolution happens by selection on existing genetic diversity. Therefore, if you want to evolve faster, you need to generate diversity faster so that the environment can select upon it. This means you want to generate a lot of beneficial and functional heterogeneity very quickly and then hold onto it, while trying to avoid deleterious mutations.
Traits that increase evolvability are co-associated with advantageous diversity that they helped generate, so they get selected for5. Over time more evolvable organisms adapt faster to changing circumstances and fill newly available niches before any competitors. This process is independent of any specific environment, so it’s universal. Traits that encourage faster evolution are selected for.
The basic version of evolvability is enhancing how quickly you accrue diversity by tweaking mutation rates and encouraging recombination. Every organism precisely tunes mutation rate to hit the sweet spot where you’re gaining the right amount of diversity but not breaking too many things6. Recombination happens via sexual reproduction and horizontal gene transfer, but it’s limited by the existing pool of diversity so there’s an upper cap to how much diversity you can gain. Those are the easy evolvability adaptions.
The advanced version of evolvability is shaping the underlying systems so that random perturbations are more likely to be adaptive and less likely to be deleterious. This has deep implications for the basic way that biological systems work.
Reliability vs. Robustness
In engineering, we think a lot about response to perturbation. You design things to not diverge from specs even in noisy conditions. This is reliability - you want your system to keep functioning the same way even as individual pieces degrade7. But eventually you’ll hit a failure point and the entire system will fail dramatically.
Reliability is not a good feature for an evolvable system. An evolving organism should change fluidly as individual components are swapped out or added, with variable but still functional outputs coming from small genetic changes8. Evolvability wants genetic diversity to translate well into phenotypic diversity, without significantly disrupting the overall system.
That last part is important - an evolvable system needs to be robust. If some parts stop doing their original job, other parts will pick up the slack to keep the organism alive. This robustness increases the space of mutations that are available, opening up the greatest diversity of genotypes and phenotypes. Not only does this mean you’ll get more good mutations, but you’ll also be more capable of holding onto a pool of neutral or weakly negative mutations that might become advantageous if the environment changes.
These two design principles seem opposed. How can you have a system that is both translatable and robust? You want your organism to survive the maximum possible range of mutations, but you want all of those mutations to have real, phenotypic effects.
Signaling Networks
To me, the best examples of robust and translatable systems are eukaryotic signaling networks. These do a huge amount of data processing in higher organisms, controlling gene expression - and thus cellular behavior - in every cell in our bodies.
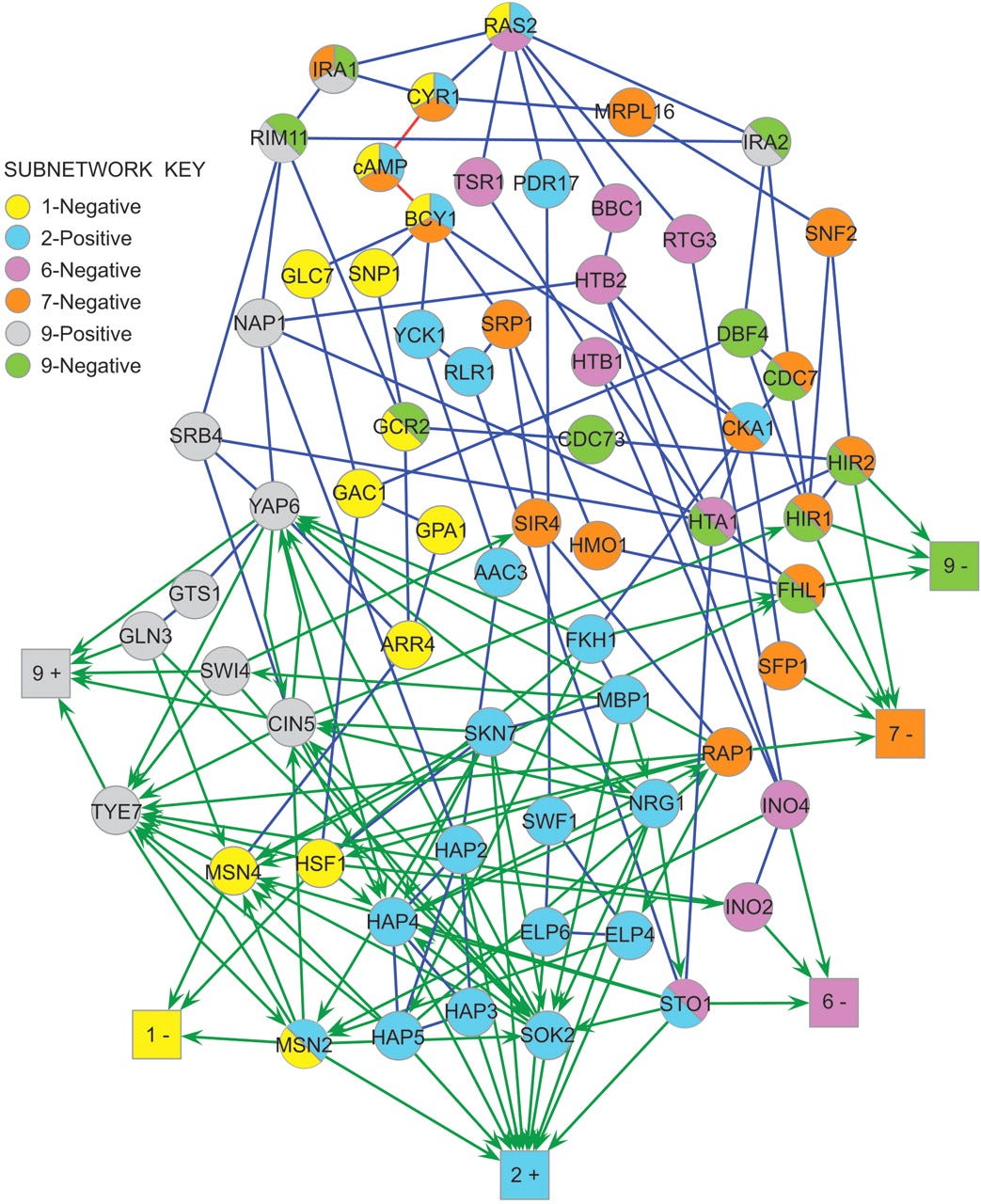
Here’s a graph representing signal transduction in the yeast Ras-cAMP signaling network9.
This is somewhat small as these kinds of networks go10, and it looks a lot like a recurrent neural network. Indeed, modelling it as a neural net is a recent and successful approach to understanding how these systems compute biological signals11.
Beyond wasting hundreds of PhD-years of time and fueling the reproducibility crisis, the complexity and feedback in these networks means that very few nodes are truly essential for any given response. However, a change in any node will likely cause some shift in every output.
This system is a nightmare to try to understand, but fabulous for evolvability - any given change will have a phenotypic effect across multiple outputs, but is unlikely to completely break anything12. Combine that with sexual recombination to mix-and-match different mutations and you’ll maximize the adaptive potential of what diversity you have.
Evolvability is not the only reason that mammalian signal processing is complex, since it also the system harder for parasites to hijack13 and probably other things too. However, I think that a primary reason for this complexity is improved evolvability.
Furthermore, biology won’t always converge on something like a recurrent neural net as the best way to do signal processing if it doesn’t need the extra evolvability. Bacteria primarily use pretty simple two-component systems for regulation, and they have some fantastically complex behavior that arises from much simpler one-to-one networks14.
The differences arise because bacteria and mammals exist in very different evolvability niches. They have very different strategies for acquiring and storing diversity.
Genetic Architecture
To dive into this idea further, let’s compare the evolvability strategies of E. coli and Homo sapiens, then talk about how that determines what their genomes look like.
E. coli live in huge populations, divide quickly and acquire diversity in response to a changing environment. They do this by relying on spontaneous mutations and by picking up free-floating environmental DNA. This strategy has a low hit rate, but across large populations you’ll usually end up with something adaptive15.
On the other hand, Homo sapiens live in small populations, divide slowly and store diversity for a long period of time. We don’t reproduce fast enough to generate selection in response to an environmental pressure, instead banking rare occurrences of diversity so there’s an existing pool to be selected upon when necessary.
These differing evolutionary strategies shape an incredible number of the basic traits of these organisms. E. coli need to be able to divide very quickly when times are good, and therefore reproduce clonally and keep their genome size small. Humans reproduce so slowly that genome size isn’t particularly limiting16, but we store a lot of diversity and mix and match it through sexual reproduction. Even though we only have five times as many genes as E. coli, our genome is a thousand times larger.
Much of that extra DNA isn’t functional. Almost all of the human genome is a messy “junk” of transposons, broken paralogs and regulatory elements, recombining and drifting without being essential or even especially important. Most of this DNA gets transcribed anyway17, even if it doesn’t do anything18. It just sits there for tens of thousands of years, accumulating and storing diversity. Every now and then an ‘orphan gene’ pops out of this morass of DNA, with no homologs in other species19. The same thing happens with regulatory elements, often directly caused by freely hopping transposons20.
While orphan genes are cool, they are rare. But a few billion bases not under selection is a lot of space for random things to happen. More common is that a gene or regulatory element will duplicate and remain mostly intact, drifting far enough to create another member of the same family while never fully becoming nonfunctional. Then it can fulfill a similar function as the original protein in a different context, and the process is more likely to happen again since there are more versions to duplicate. The incredible diversity of GPCRs are a great example of this happening over evolutionary time21.
There are more examples of evolvability literally everywhere in biology, but I just want to hit a few more high points that lie in my area of expertise.
Introns provide a large target so domains can get easily swapped between proteins. Most recombination events anywhere in several-thousand-base intron will produce a properly spliced protein with a new combination of domains. Without introns, you need single-base precision in your recombination.
The nucleus creates physical separation of transcription and translation, allowing those steps to be differentially regulated in eukaryotes. This is one more lever that can be used to generate diversity - if it is beneficial to upregulate a specific developmental marker at a specific time to grow larger lungs or something, that can now happen in multiple ways.
The above is also true for post-translational modifications.
Plants have a suite of adaptations to encourage genome plasticitiy22, enabling interspecies hybridization and tolerance of variable genome ploidy. For example, invasive weeds often have incredibly plastic genomes that allow them to adapt quickly when invading a new niche. They hybridize with everything and their genomes balloon, then the bloat slowly gets purged down to something well-adapted for the local environment23.
Bacteria package similar genes together into operons so they can be treated as a functional unit that is easy to regulate as a unit, meaning that one mutation can easily shift the activity of an entire pathway.
Viruses are the masters of evolvability through incredibly rapid replication & rapid base-pair optimization. They often stack multiple genes atop each other in different coding frames to reduce genome size24.
I’m sure that there are similarly fundamental systems across biology that are heavily influenced by evolvability. Some examples might be how morphogen gradients work and how brain architecture is encoded. But I’m an expert in bacterial genetics and have dabbled in aging research25, so I don’t feel qualified to speculate on those topics.
It’s hard to think about the effect size of any evolvability adaptation. But most of them open up whole new dimensions of diversity, so the outcome is rather nonlinear. We’d also expect more evolvability adaptations in larger organisms that replicate more slowly. They need all of the advantages they can get to keep up in the red queen’s race.
Engineering Evolvability
I hope you will agree with me that evolvability is not just a knob to turn up or down, but a fundamental feature of biology that must be taken into account when engineering life. That insight doesn’t necessarily limit what kinds of engineering we can do. If anything, knowledge of evolvability gives us a clearer view of the implications of biological research.
For example, one of the main limitations on the speed of animal embryonic development is the time it takes for genome duplication26. If we want to engineer organisms with faster gestation times, reducing the genome size by taking out all of the nonfunctional DNA might be a good way to do that. By understanding why that DNA exists, we know what we’re sacrificing. It probably wouldn’t be very effective to do selective breeding on cattle with a minimal genome because we’ve removed a lot of their banked diversity.
Similarly, the complexity of mammalian transcriptional regulation isn’t vital for functionality, but if you engineer a simple and direct synthetic signaling network, it will be much more likely to break instead of adapt. This is probably a good thing if we’re growing houses and you’d rather have something die instead of grow out of control. In order to engineer biological systems that are reliable instead of robust, decreasing evolvability is a good thing.
On the other end of the spectrum, directed evolution is an important way to develop new biological technology27. Optimizing evolvability has revolutionized this field, yielding more powerful results by increasing the search space28.
These developments haven’t reached whole-organism engineering - but that’s where they’ll be the most impactful, especially when we start synthesizing genomes. The first big target is likely to be biomanufacturing, since none of our bioproduction organisms evolved to do well inside giant steel tanks full of industrial solvents29, fed rich nutrients to continually produce bioproducts. We’ll need to target evolution towards that end. The more elements of evolvability we include in that process the better the result will be.
This principle is true beyond bioreactors. If you want to take life to space, you’re going to run into environments and raw materials that no life on earth has evolved for, such as abundant perchlorates and unshielded ultraviolet radiation30. These are novel challenges to biology, and we’ll need to build organisms for rapid evolution to spread life to the stars.
Just as important as accelerating evolution is learning how to slam on the brakes. If you can slow evolution to near-zero, you prevent adaptation away from design parameters. We need to biocontain strains before they can be used outside of the lab31, and decreasing mutation rate, simplifying genome architecture and removing recombination mechanisms are all important steps towards that goal. Next-generation biocontainment might even rely on low evolvability as a primary way to make an engineered organism noncompetitive in the wild.
Life Understanding Life
Finally, I think that evolvability is an essential part of understanding biology. The best way to understand the purpose behind a given trait is to understand how it evolved. The best way to understand many traits together is to understand how their interactions shape their evolution and capacity to adapt.
Many synthetic biologists repeat the Feynman quote “What I cannot create, I do not understand.” Part of engineering life is not just being able to design it de novo, but to understand and engineer the actual forces that governed the creation of life as we know it. Synthetic biology as a discipline should consider evolvability of both natural and designed systems, and always be aware of the trade-off between reliability and robustness.
Thanks to Merrick Smela, Anjali Kayal and Sarah Scheffler for providing feedback on this article.
Famous essay on the topic: Dobzhansky, T. Nothing in Biology Makes Sense except in the Light of Evolution. The American Biology Teacher 35, 125–129 (1973).
https://www.pioneer-labs.org/
We don’t currently have great ways to control bioengineered strains in nature. If we ever want to use biotech outside of big steel tanks we need to figure out how to “biocontain” them. I think part of the solution is limit the organsism’s evolution so it cannot easily escape from control.
It’s pretty unlikely that adaptations that make a house better at reproducing also make it into a better house. Unless we do selective breeding - but that’s just a kind of bioengineering.
Earl, D. J. & Deem, M. W. Evolvability is a selectable trait. Proceedings of the National Academy of Sciences 101, 11531–11536 (2004).
This often happens in response to the environment. Metzgar, D. & Wills, C. Evidence for the Adaptive Evolution of Mutation Rates. Cell 101, 581–584 (2000).
Plumbing or electrical networks are both good examples. Even if subcomponents of the whole change or fail, you want the rest of the system to continue functioning as intended.
A good example is Darwin’s finches. Small changes in genetics led to large changes in beak morphology, but the robustness of beak development means that they’re all still functional beaks.
al-Mosleh, S., Choi, G. P. T., Abzhanov, A. & Mahadevan, L. Geometry and dynamics link form, function, and evolution of finch beaks. Proceedings of the National Academy of Sciences 118, e2105957118 (2021).
Carter, G. W., Rupp, S., Fink, G. R. & Galitski, T. Disentangling information flow in the Ras-cAMP signaling network. Genome Res. 16, 520–526 (2006).
I almost wanted to use this image on the human signaling networks relevant to tumorigenesis, but decided it was unnecessarily busy.
Seeger, M., Longden, J., Klipp, E. & Linding, R. Deep Hidden Physics Modeling of Cell Signaling Networks. Curr Genomics 22, 239–243 (2021).
1.5% of point mutation SNPs cause genetic disease, and around 3% of nonsense mutations - which are a (much more damaging) subset of point mutations. These numbers are far lower than I would expect a priori. Chen, R., Davydov, E. V., Sirota, M. & Butte, A. J. Non-Synonymous and Synonymous Coding SNPs Show Similar Likelihood and Effect Size of Human Disease Association. PLOS ONE 5, e13574 (2010).
Del Giudice, M. Invisible Designers: Brain Evolution Through the Lens of Parasite Manipulation. The Quarterly Review of Biology 94, 249–282 (2019). I learned about this theory here: Alexander, S. Maybe Your Zoloft Stopped Working Because A Liver Fluke Tried To Turn Your Nth-Great-Grandmother Into A Zombie. Slate Star Codex https://slatestarcodex.com/2019/08/19/maybe-your-zoloft-stopped-working-because-a-liver-fluke-tried-to-turn-your-nth-great-grandmother-into-a-zombie/ (2019).
Capra, E. J. & Laub, M. T. The Evolution of Two-Component Signal Transduction Systems. Annu Rev Microbiol 66, 325–347 (2012).
E. coli mutate once every thousand divisions. That means if you grow a single cell (a colony) to 6*10^12 cells, you have on average mutated every base in the E. coli genome. That’s about half a liter of saturated culture depending on your media.
Source for E. coli mutation rate: Lee, H., Popodi, E., Tang, H. & Foster, P. L. Rate and molecular spectrum of spontaneous mutations in the bacterium Escherichia coli as determined by whole-genome sequencing. Proceedings of the National Academy of Sciences 109, E2774–E2783 (2012).
Hidalgo, O. et al. Is There an Upper Limit to Genome Size? Trends in Plant Science 22, 567–573 (2017).
Pertea, M. The Human Transcriptome: An Unfinished Story. Genes (Basel) 3, 344–360 (2012).
Eddy, S. R. The ENCODE project: Missteps overshadowing a success. Current Biology 23, R259–R261 (2013).
Singh, U. & Syrkin Wurtele, E. How new genes are born. eLife 9, e55136.
Sundaram, V. & Wysocka, J. Transposable elements as a potent source of diverse cis-regulatory sequences in mammalian genomes. Philosophical Transactions of the Royal Society B: Biological Sciences 375, 20190347 (2020).
Strotmann, R. et al. Evolution of GPCR: change and continuity. Molecular and Cellular Endocrinology 331, 170 (2010).
Michael, T. P. Plant genome size variation: bloating and purging DNA. Briefings in Functional Genomics 13, 308–317 (2014).\
Leitch, A. R. & Leitch, I. J. Genomic Plasticity and the Diversity of Polyploid Plants. Science 320, 481–483 (2008).
Shimizu-Inatsugi, R. et al. Plant adaptive radiation mediated by polyploid plasticity in transcriptomes. Molecular Ecology 26, 193–207 (2017).
Clements, D. R. & Jones, V. L. Rapid Evolution of Invasive Weeds Under Climate Change: Present Evidence and Future Research Needs. Frontiers in Agronomy 3, (2021).
DiMaio, D. Viruses, Masters at Downsizing. Cell Host & Microbe 11, 560–561 (2012).
Hur, J. H., Stork, D. A. & Walker, D. W. Complex-I-ty in aging. J Bioenerg Biomembr 46, 329–335 (2014).
Rhind, N. DNA Replication Timing: Biochemical Mechanisms and Biological Significance. Bioessays 44, e2200097 (2022).
Arnold, F. H. Design by Directed Evolution. Acc. Chem. Res. 31, 125–131 (1998).
Packer, M. S. & Liu, D. R. Methods for the directed evolution of proteins. Nat Rev Genet 16, 379–394 (2015).
Molina, R. S. et al. In vivo hypermutation and continuous evolution. Nat Rev Methods Primers 2, 1–22 (2022).
Timson, D. J. The roles and applications of chaotropes and kosmotropes in industrial fermentation processes. World J Microbiol Biotechnol 36, 89 (2020).
https://www.authorea.com/users/795897/articles/1116574-engineering-bacteria-for-environmental-release-regulatory-challenges-and-design-strategies